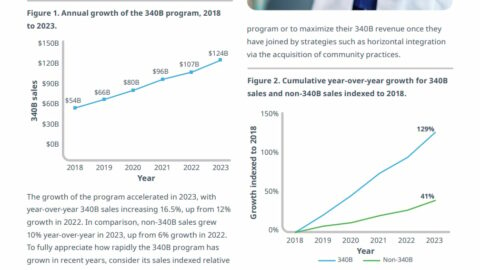
The Wall Street Journal covered, with some depth, the trend of biosimilars launching with two prices: a low price designed to be appealing to consumers (and, I guess, media) and a high price for the PBMs.
It was an idea that first started to come into vogue with biosimilar insulin (though there was an example even earlier, with a PCSK9 drug), and we’re now getting a sense of how the strategy is working in practice: low-priced versions are by and large not that available.
This is not necessarily new, but the coverage comes at an interesting time, where PBMs are suddenly in the hot seat. There are a couple of important takeaways.
The first is that the harm here is being very clearly centered on one group of patients: those with coinsurance, which is generally based on list price, not net price after rebates. So even though it might seem like insurance coverage of a branded medicine should eliminate patient affordability concerns, there are plenty of patients that would benefit directly from preferential coverage of a cheaper option.
That’s really a critical frame in telling this story, and I’m glad the WSJ used it.
The second is that PBM messaging here is weak and implicitly ignores patients, which might have been OK a couple of years ago but now seems pretty reckless. An Express Scripts exec told the outlet that they “make decisions on what is the lowest net cost to a plan sponsor,” which is code for “patients can go pound sand.”
Not really a good look for that industry.
Politico has coverage of an AARP poll set to drop later this week. I’m not sold on the conclusions — 42% of seniors think drug prices will be “extremely” or “very” important in how they vote, and 39% are educated on the IRA. Both of those numbers seem inflated, probably by design. I’ll have more when more data drops.
Monica Bertagnolli won confirmation as the director of the National Institutes of Health, though it doesn’t seem like that’s going to quiet Bernie Sanders.
The FTC is continuing its work of getting rid of questionable Orange Book patents, telling 10 companies to take a real hard look at 110 patents — mostly around delivery devices — or else. It’s not obvious how big or widespread the problem is, but it’s generating headlines for the FTC, which i suppose is a win for the agency.
The FDA’s Peter Marks weighed in on commercial perils facing gene therapy, telling Milken attendees, per Politico, that, “If we don’t get this right in the next 10 years, I think we’re going to have a real problem of crisis proportion of having the technology to address a lot of these diseases but not being able to deliver them because health care payers have decided that it’s more important that people can get their annual flu vaccines.” On the one hand, he ain’t wrong. On the other hand, this is a classic example of a place where it’s a lot easier to define the problem than solve it.
If this email was forwarded to you, and you’d like to become a reader, click here to see back issues of Cost Curve and subscribe to the newsletter.