Hoo boy … is there ever a lot on my mind. Let’s get going.
Late-breaking news: the FDA has green-lit Florida’s plan to import medicines from other countries.
I’m on the record as an importation skeptic, and I assume that this will be a far bigger political talking point than it will be an effective way to drive prices down, and nothing I’ve seen this morning is dissuading me from that belief.
Indeed, the New York Times must-read coverage of the move is chockablock with healthy skepticism. Actually implementing something means getting past inevitable industry lawsuits, restrictive commercial arrangements, and the opposition of the Canadian government.
But it’ll make for great soundbites.
I’m a couple of days away from getting on an airplane and heading to San Francisco for the J.P. Morgan Healthcare conference. As I mentioned earlier this week, I’d really love to say hello to any readers who will also be there, and happy to share my whereabouts to make a connection. Please reach out.
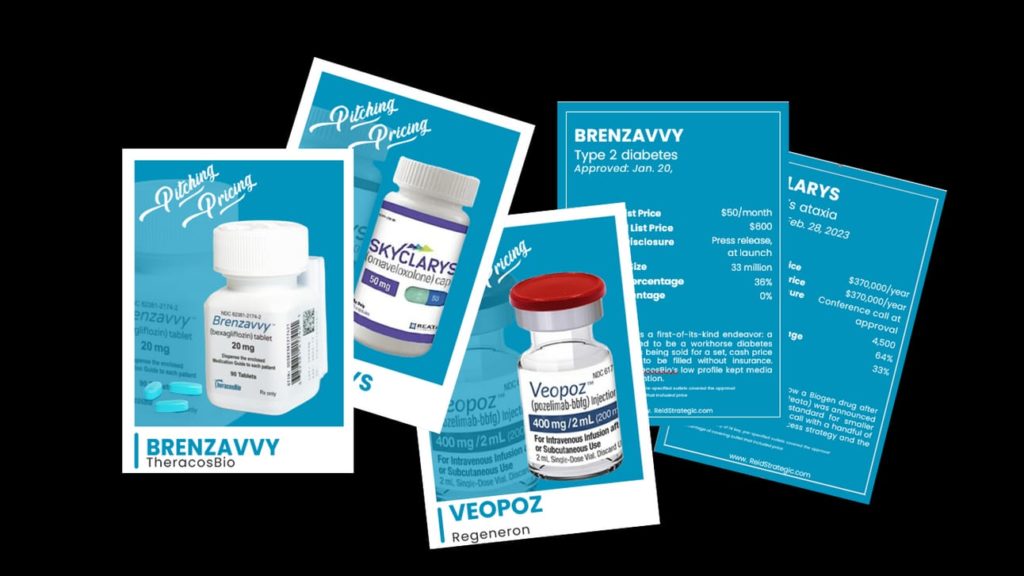
I’ll show up at the St. Francis with a satchelful of “Pitching Pricing” cards. The mock baseball cards, for 2023-launched meds, include each drug’s “stat line”: not only the price, but the patient population, how the price was disclosed, how media covered each pricing announcement, and more.
It’s a little goofy, but I want to emphasize that communication around how a medicine enters the market is a highly variable endeavor, and it should not be thrown together without a broader strategy and without thinking of the myriad of audiences — not just payers and investors but patients, advocates, physicians, policymakers, and others — for whom price is a key economic variable, a defining reputational marker, or a crucial component of access.
If you want your own set of “Pitching Pricing” cards but you won’t be at #JPM24, drop me a line with your physician address. And, naturally, if you need help talking price and value around a 2024/2025 launch, I’m here to help.
So — yeah — I’ll be at J.P. Morgan next week, and I’m grateful for those of you who have reached out. Looking forward to connecting with many of you.
As you all know, it’s a weirdly anti-climatic meeting. There may be some big news made ahead of the conference, but it’s not like there will be a steady string of breaking items. Instead, it really does become about checking the industry’s temperature on dozens and dozens of issues.
Here are the trends I’m chasing around drug pricing:
The Inflation Reduction Act: Companies pretty much ignored the issue entirely during the 3Q earnings calls, so I’m curious who will say what next week. I get that there isn’t much to say about implementation of the legal battles, but the way that the IRA is impacting business decisions remains an important and under-discussed element of all of this, so I’ll be paying attention to the tea leaves there.
Legislative Priorities: Look, I know nothing will (probably) happen in DC this year, but that doesn’t mean that companies won’t (or shouldn’t!) use their platform to talk up policies that make sense. Will anyone mention PBM reform? TROA? ORPHAN Cures? Other IRA fixes? The time to put down markers is now, but will anyone take the opportunity?
Whither Rebates: The rebate dialogue seems to be headed in two different directions. On the one hand, there is a growing consensus that we’d be better off if the rebate system just got blown up. On the other, companies are employing increasingly complex rebate-centered strategies to maintain volume in competitive spaces. I’m hoping next week gives some hints as to what view of the future will dominant in 2024.
Value: I believe that, in the future, every drug will launch with its value clearly defined and that forward-thinking companies will make efforts to begin fleshing out the underlying value story ahead of approval, in the same way that the clinical story behind a given medicine is well-understood before a medicine is launched. So I’ll be tracking on any company that includes value details in its corporate presentation.
What Else?: Let me know what I’m missing, and I’ll tune my radar accordingly next week.
I mentioned ORPHAN Cures above, and here’s an op-ed from BIO’s chairman, Ted Love, pushing for that legislative fix around the IRA’s treatment of rare diseases.
ICER’s new president, Sarah Emond, is going on a listening tour. If you want time with Sarah, you can request it here.
ICER, BTW, is tackling Geron’s therapy for myelodysplastic syndrome as its next review.
Cost-plus is the new AI. Which is to say, every company in the drug supply chain has to nod to the concept, regardless of whether they’re actually doing anything remotely cost-plus-y. Today’s case-in-point: Walgreens.
Reality check: the average American is not remotely tracking on most of the important health policy issues that take up so much space in my brain. My evidence here is that the Washington Post’s columnist on health issues, Lena Wen (who writes for the average American, not the health wonks), published a list of her top 10 policy issues for 2024, and they don’t align whatsoever with what I consider to be the actual top 10 issues.
bluebird bio said in a regulatory filing that it signed a second outcomes-based agreement around its sickle-cell gene therapy, Lyfgenia, and that 200 million lives are now covered by an outcomes-based agreement. No word on specifics, though. (h/t to Endpoints)
If this email was forwarded to you, and you’d like to become a reader, click here to see back issues of Cost Curve and subscribe to the newsletter.