If you want to search Cost Curve back issues or link to anything you read here, the web links and archive are online at costcurve.beehiiv.com. You can subscribe there, too.
UnitedHealth Group is, objectively, having a crappy year. It was hacked, resulting in a fiscal armageddon of sorts for providers. Andrew Witty got hauled before Congress. The FTC is suing them for anticompetitive behavior.
And, to top it all off, people are using health care at higher-than-expected rates. (If there is one thing that insurance companies hate, it’s when people actually use their insurance.)
One would think that would be catastrophic for the company’s business, but … Eh. United is fine. Quarterly numbers are out today, and they’re eye-popping, as usual. Revenue exceeded $100 billion. (That’s a one followed by eleven zeros.) Earnings from operations were more than $6 billion. Not bad work, if you can get it.
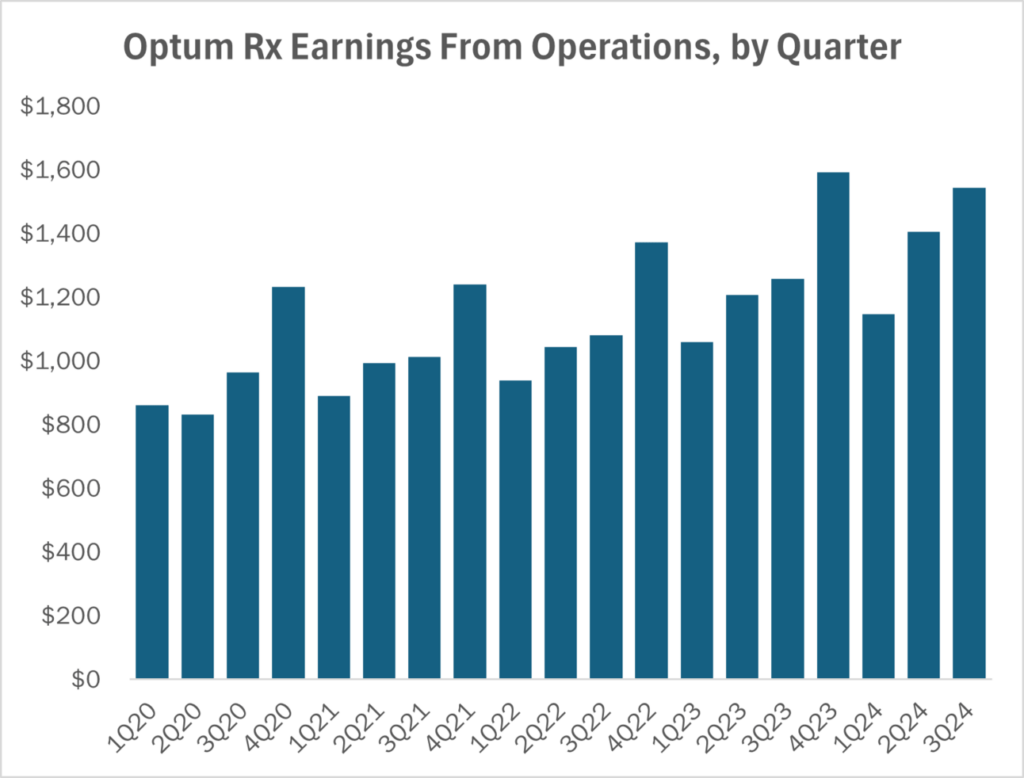
Part of what is propping up the whole enterprise is the Optum Rx business, which is where the company’s PBM is located. Revenue there was $34 billion for the quarter. Earnings from operations were a tick more than $1.5 billion, about 23% more than the company saw in the third quarter of last year.
If you’ve been reading Cost Curve for any length of time, you know what’s coming next.
While I’m not opposed to companies providing services and profiting, the incredible quarterly numbers again raise the question: what, exactly, is UnitedHealth doing to earn that $1.5 billion? Consumers aren’t happy with prices or access, and United’s direct customers — employers — are dealing with ever-higher prices.
In other words, the only real winners in all of this … are the PBMs.
Look, I don’t want to pivot to media criticism here, but I’m shocked that this Barron’s story isn’t getting linked to all over the place.**
It’s about the ways that PBMs extracted rebates from opioid manufacturers in return for formulary placement that encouraged use, thereby allowing PBMs to grab a larger cut of the opioid dollars.
I don’t have a lot of commentary here: just go read the piece. It is the best thing written on PBMs this year (yes: better than the NYT piece).
The PBM defense here is that the documents Barron’s used are taken out of context, and that PBMs have done a great deal to ensure the responsible use of pain meds. It’s possible — probable, really — that two things are true. PBMs were in deep on the opioid business via rebates, and there were a lot of other PBM programs that pushed in the right direction.
In a lot of ways, that’s the PBM story in a nutshell: PBMs do have, and use, tools to make health care better. But they also have a huge incentive to use rebates in a way that maximizes their profits in a way that is, shall we say, agnostic to clinical considerations.
The question with PBMs is whether the good stuff can be cleaved from the questionable stuff.
** Seriously: Can anyone point me to a newsletter or other media source that cites to the Barron’s piece? This tree is way too big to fall in a forest without anyone hearing it.
Adam Fein is out with a provocative new post that looks at PBM satisfaction data. Adam’s thesis is that plans/employers really aren’t as upset with PBMs as the conventional wisdom would suggest.
Rather, plans are perfectly happy with low transparency and big rebates, as long as they can use those rebates to buy down premiums … the only number that matters, by Adam’s calculus. If that means higher costs for medicine-using employees, well, them’s the breaks. “Plan sponsors know precisely what they are doing,” he writes.
I’m not sold on the idea that employers are playing 3D chess on all of this (Mark Cuban, too, is skeptical). But Adam’s conclusions fit the data. I suppose we’ll get some answers in the next couple of years. If employers and plans don’t defect in meaningful numbers, Adam will have called it.
ELSEWHERE:
Happy Open Enrollment Day. There’s plenty of good, news-you-can-use-style coverage out there, and I wish everyone the best of luck in navigating the rapidly evolving choices this year.
I love this: CMS claims to be doing evidence reviews as part of its IRA price control process, but they’re super-vague on how that review works. So three of my favorite health economists published a great piece in Health Affairs explaining how CMS should be operating around evidence reviews. It also makes the point that doing this right would probably take more time than CMS is allotted, which is more evidence that the law ended up being half-baked.
BIO and PhRMA are both asking HRSA to rethink its opposition to the “rebate model” in 340B. I assume HRSA is going to keep its head in the sand, but the letters from the two groups make it plain that this matter is going to have to be settled definitively, one way or another. That is code for “cue the lawyers.”
Lord, this is dumb. Some researchers have determined that, because of vial size issues, just under 6% of each Leqembi dose is wasted. That’s all well and good, but then the researchers went on to figure out what that would “cost” Medicare, assuming everyone who could get the drug did get the drug: $300+ million. That’s one hell of a leap for a medicine with $40 million in total sales in the second quarter. What’s worse, the media were all over the story, without providing any context. Sigh.
I’m not following the dynamics in Europe closely enough to know whether this Politico story holds water. It’s based on a report that says that drug spending is up big in European nations, largely because of price. I’d be grateful for anyone who can send along a compelling theory for what’s going on.
Perhaps the biggest misunderstanding around the “negotiations” provision of the IRA is that it was intended to make life better for patients. It wasn’t. It was designed to save the government money, which could be invested in any number of things, including electric vehicle incentives and the $2,000 out-of-pocket cap. It wasn’t really designed to meaningfully lower consumer costs on its own. That’s a point that is made eloquently in this IQVIA analysis that looks, drug-by-drug, at the likely patient impact of the first 10 price controls.
Another datapoint in the most important story in American health care (the death of retail pharmacy): Walgreens is going to shutter 1,200 stores.
This is an interesting one, philosophically: makers of inhaled medicines are moving to more environmentally sound methods of delivery, which means extended patent life on those products. This mammoth Politico story spins that as a bad thing, but it never asks an interesting question: in theory, anyone could have invested a billion dollars and come up with the improved devices. Why throw shade at the companies that actually made that investment?
Thanks for reading this far. I’m always flattered when folks share all or part of Cost Curve. All I ask is for a mention or tag. Bonus points if you can direct someone to the subscription page.