For fans of 340B and/or “Where’s Waldo”: I’ve hidden a single 340B reference in this newsletter. Can you find it?
INFLECTION POINT/ IQVIA Quantifies Innovation … And Points to Some New Trends
You all should probably stop what you’re doing and go read IQVIA’s new “Global Trends in R&D 2025” report right now. I could summarize it for you, but — chances are — you’re going to find a nugget in the report itself that I would miss.
It’s got M&A details, broken down in every which way. Trial failure rates from all angles. Launch data. Trial complexity. You name it, it’s probably in there.
It’s quite the resource.
So, instead of trying to give you all a SparkNotes version, I figured I would instead show my three favorite images from the report to give a flavor of where we are right now from an innovation POV.
First, here is what I consider to the topline: how much money is big pharma pouring into R&D? The answer is “a lot,” both in absolute terms and relative to sales. No other industry is making this kind of investment.
Second, China is rising, Europe is falling. It’s dramatic. I don’t want to wrap myself in the flag here as much as ask the obvious questions: What is China doing right? What is the EU doing wrong? And are American policymakers pushing the United States toward more of a Chinese innovation ecosystem or a European one?
Third: Is industry maybe getting better at research? The spike in successful Phase 3 trials last year is encouraging, though these data are noisy (and Phase 2 remains kind of a house of horrors).
Curious to hear what catches your eye here.
THE ARC/ Pharm to Table
I guess the theme for the week is “pharm to table.” I wrote about Novo’s latest forays (and the limits thereof) Tuesday, but that wasn’t it: The topic also appears in this week’s edition of the august New England Journal of Medicine.**
The NEJM paper, by public-health oriented folks at Brown, is mostly an exercise in pearl-clutching about the idea of pharmaceutical companies linking up with telehealth companies, complete with a scary headline: “Partnerships between Pharmaceutical and Telehealth Companies — Increasing Access or Driving Inappropriate Prescribing?”
My read of the heavily caveated paper is that there are two big areas of objection
The arrangements between pharma and telehealth companies illegally violate anti-kickback statutes, and/or
The telehealth companies are going to provide terrible care, mostly via inappropriate prescribing.
Objection 1 is certainly popular (some members of Congress are sniffing around the idea, too) but has always felt tenuous. My assumption is that the existing arrangements are some of the most over-lawyered contracts in the history of the pharmaceutical industry.
I don’t know that for certain, but I do know that the legal departments of Pfizer and Lilly are not full of forgiveness-not-permission cowboys. The idea that there is a seam showing feels unlikely.
I understand the allure of Objection 2, as well. Telehealth does not have a spotless record, and the whole idea of “telehealth” is hugely heterogenous. If you want to find examples for bad, or even borderline, behavior, you probably don’t have to look very hard.
But, again, my guess is that the folks working with Lilly and Pfizer are buttoned-up. (See the point about being over-lawyered, above.) Those companies are not partnering with fly-by-night pill mills. The reputational cost would be too high. I don’t think there are skeletons in those closets.***
Indeed, I spent the day Tuesday at BMO’s Obesity Summit, where a parade of pharma companies made their best pitches about the future of obesity. Also presenting was Ro, one of Lilly’s partners, and their spiel — which centered around a laser focus on their customer — was just as compelling as any of the innovators.
Because that’s the counterpoint: At the end of the day, “pharm to table” only succeeds to the extent to which it can deliver for the patients.
I don’t want to whistle past the graveyard here. These arrangements do carry legal risks. Telehealth does come with some compromises. More research would be welcome.
But is it likely those risks/compromises are practically meaningful? Is there any evidence out there that we can talk about right now without casting aspersions? I’m unconvinced.
** There is also a Q-and-A style STAT interview with the NEJM authors, which is equally unconvincing. The number of times the academics talked about the issues that “might” exist or problems that “could” arise — with absolutely zero evidence that those concerns are real — was striking.
*** If people were honestly worried about inappropriate GLP-1 use, the place I’d recommend starting would be the fringe-ier side of the med spa business, not the most legitimate side of the telehealth business. But that’s just me.
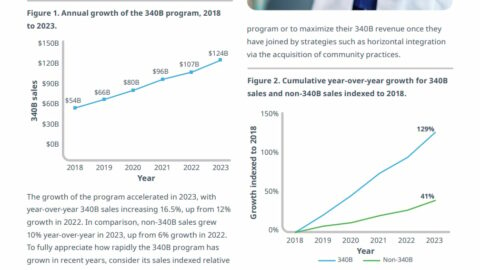
TRAJECTORY/ Launch Price Communication Report: Qalsody
Reid Strategic has gathered an immense amount of background on medicines that have been launched over the past three years, tracking when and how launch prices have been disclosed, as well as how those pricing decisions have been covered by a curated group of 13 media outlets. Those numbers have been boiled down into one-page “Launch Price Communications Reports.”
In mid-2023, Biogen won approval for Qalsody, a medicine designed for a small segment of the amyotrophic lateral sclerosis patient population: a genetically defined group of fewer than 400 patients.
Traditionally, that kind of super-targeted medicine garners a higher-than-average price tag, but Biogen opted to price the medicine in line with other therapies for ALS, even though those other treatments had efficacy concerns and were aimed at a far larger population. Qalsody ended up being in the bottom half of 2023 approvals, list-price-wise.
But Biogen didn’t trumpet the price, and while the approval was widely covered, only one of the media outlets tracked by Reid Strategic reported on the price. The lesson: Even pricing decisions that seem like they would be praiseworthy are unlikely to be noticed without a proactive effort.
QUICK TURNS/ PBM Reform Coming, for Real This Time, Plus How to Think About Tariffs
Rep. Buddy Carter had some interesting disclosures at yesterday’s “Health Next Summit” from The Hill.
Here’s Carter on PBMs: “We have a commitment from the speaker and from leadership that these bills … will be put into the reconciliation bill. They will save us some money that can be used as pay-fors. They’ll save the federal government some money, but this will be an opportunity for us to get true PBM reform. It is not the end. It is only the beginning of PBM reform.”
And 340B: “I would be remiss if I didn’t mention the 340B program at this point. That’s a program that has gone amuck and has evolved into something that it was never intended to be. It’s a vital program for FQHCs, for federally qualified health centers and for rural hospitals, but it has really gotten and grown to a point where it needs to be reformed again and we need more transparency in it.”
ELSEWHERE:
If you were a policymaker inclined to approach the topic of tariffs on pharmaceuticals thoughtfully, you’d read every word of this Health Affairs Forefront piece by Mark McClellan and colleagues and take their advice to heart.
Money directed at public health isn’t an expense. It’s an investment. And in the case of HIV prevention, it’s an investment that pays off in a relatively short amount of time. That makes cuts to those kinds of programs economically puzzling. The whole argument is detailed well in this STAT op-ed.
Cost Curve is produced by Reid Strategic, a consultancy that helps companies and organizations in life sciences communicate more clearly and more loudly about issues of value, access, and pricing. We offer a range of services, from strategic planning to tactical execution, designed to shatter the complexity that hampers constructive conversations.
To learn more about how Reid Strategic can help you, email Brian Reid at brian@reidstrategic.com.