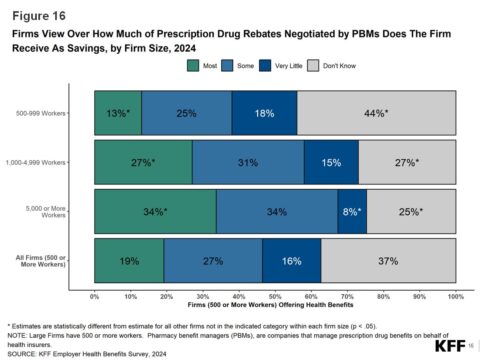
There is a lot to talk about this morning, but I don’t want to lose track of the fact that my social media manager scored the game-winning goal in her first college game.
OK. First, we need to talk about the FTC and PBMs.
I suppose the best place to start is with the lawsuit filed Friday by the FTC against the three largest PBMs and a number of affiliated companies (mostly GPOs … more on them in a bit).
The FTC’s press release is pretty clear about what the agency is upset about:
Today, the Federal Trade Commission brought action against the three largest prescription drug benefit managers (PBMs)—Caremark Rx, Express Scripts (ESI), and OptumRx—and their affiliated group purchasing organizations (GPOs) for engaging in anticompetitive and unfair rebating practices that have artificially inflated the list price of insulin drugs, impaired patients’ access to lower list price products, and shifted the cost of high insulin list prices to vulnerable patients.
While the suit is only going after the PBMs, an associated statement from the deputy director of the agency’s Bureau of Competition Deputy Director also put manufacturers on notice, though it’s not clear if this is double-secret probation or what.
Indeed, the FTC’s ultimate goal is all of this is a little hazy, in part because the actual lawsuit isn’t public yet. That should happen today, so maybe there will be more clarity tomorrow. The suit is not a big federal deal and will instead take place in FTC’s administrative court.
I’ve looked at a lot of the coverage, and it doesn’t seem like anyone has a good sense of the endgame here. At a minimum, though, it’s not a good look for an industry that’s working like h*ck to neuter or kill additional regulation.
In some ways, the FTC should get some credit for moving ahead with their suit right after Express Scripts filed a different lawsuit trying to get the agency to retract its not-very-supportive analysis of the PBM industry. Last week, I wondered if the Express Scripts effort was, perhaps, an effort to get the FTC to think twice about moving forward with litigation.
If that was indeed an aim: mission not accomplished.
As I dug into this over the weekend, it feels more and more like the suit that Express Scripts filed was designed as a cri de coeur — a display of faith to its clients and supporters — rather than a serious legal challenge.
My opinion here was heavily influenced by this blog post from Antonio Ciaccia and his team at 46Brooklyn that just tears apart the PBM-commissioned research that serves as the foundation of the Express Scripts action. It’s hard to go through the 46Brooklyn takedown and conclude that the PBMs are presenting a thoughtful, defensible analysis.
I won’t go overboard in repeating the blog post’s take, but it’s pretty comprehensive. From questioning accounting standards to pointing out the way that GPOs are entirely absent to noticing that the PBMs sometimes refused to provide data to their own academic,** the post paints the picture of unserious work. Certainly not the kind of thing to build a robust legal challenge around.
That’s not to say that the FTC report doesn’t rely on circumstantial evidence of its own. But if Express Scripts hoped to gain the upper hand through more sober data analysis, I don’t think they succeeded.
** The academic in question is a University of Chicago professor named Dennis Carlton. Carlton is low-key famous for being the guy that big companies go to when they want to tamp down antitrust concerns.
ProPublica, back in 2016, estimated that Carlton had made $100 million from his consulting work. That figure was repeated again on Friday when the New York Times looked at Carlton and his ilk.
Look, I’m a consultant, too. I respect the hustle. I’m just saying that if one of the 50 largest companies in the world wanted to write a report to be used to file a suit against the federal government, I would fully expect folks to question my credibility. Especially if I was Scrooge McDucking in a swimming pool of money.
A couple of months ago, I flagged a great webinar led by UCSD’s Inma Hernadez in which Inma and her team went through their best-bet predictions for the next 15 drugs to be selected for price controls.
Now, that group has a publication out summarizing those predictions. They’re on the record for 13 of the 15 medicines, along with another seven that they say could make the final list, depending on sales numbers, generic competition, and how the orphan drug provisions of the law are interpreted.
The list itself shouldn’t be a giant surprise to folks tracking things closely, but it’s a fantastic resource. The actual announcement — due by Feb. 1 — is going to be dominated by headlines about Ozempic/Wegovy/semaglutide, but the real drama will be which medicines end up on the tail end of the list and which ones will just barely escape.
Anyway: this is one to clip and save.
Tomorrow, Novo CEO Lars Fruergaard Jørgensen is going to get hauled before Bernie Sanders’ Senate committee for a heavy sack beating. There’s not much to say here. That’s not going to stop a lot of people, particularly Bernie, from saying a whole lot.
But, at the end of the day, there’s not much Bernie can do but rant.** These are cost-effective medicines under extraordinary cost pressures. In the long term, the market is going to solve these problems (the WSJ had a great look at this on Friday). In the short term, solutions would need to be broad and creative and focus on the reallocation of government resources.
Bernie is neither patient enough for long-term solutions nor serious enough to do the hard work for quick action. He is, however, great at driving headlines.
** OK, OK. Maybe the ranting will have some impact. Oliver Barnes, writing in the Financial Times, quotes AEI’s Ben Ippolito floating an interesting theory. Perhaps, goes the Ippolito argument, Sanders is just trying to soften the ground for harsh price controls under the IRA next year, prompting a would-be Harris administration “to be especially aggressive when it sets that price”.
Elsewhere:
While we’re talking obesity, the Guardian looked at the cost-effectiveness of the GLP-1s over in Europe, citing an analysis that finds that the societal impact more than outweighs the financial cost.
STAT has a piece on a “mysterious” anti-pharma bus campaign. To STAT, it’s mysterious because we don’t know who is bankrolling it. The whole endeavor is run by Trump-adjacent individuals, and the point seems to be to raise awareness of international reference pricing approaches. The mystery to me is not so much who is funding this as much as what they hope to accomplish. A populist uprising around the importation of price controls?
Sen. Bill Cassidy has a bill that would expand the small-company exemptions in the IRA, which are due to sunset in 2028.
Curious about the agenda of those who want to fundamentally reshape how patents are handled in the biopharmaceutical industry? This op-ed from STAT gives a nice high-level overview of the pushes to come, divided into six different categories. Whether or not you like the policies, these are the battle lines.
That’s not actually the end of the list for today, but I’m running out of time, and I’m sure that you’re running out of patience, given the current word count.
So more coming this week on smoothing, some thoughts on the harrowing and illuminating experience of Endpoints’ John Carroll, and musings on a patient advocacy op-ed that, perhaps, tells the wrong story.